![The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com](https://homework.study.com/cimages/multimages/16/cms53022462554618608855.jpg)
The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com
![NiCl4] 2– is paramagnetic while [ Ni(CN) 4 ] 2– is diamagnetic - CHEMSOLVE.NET | Electron configuration, Electrons, System NiCl4] 2– is paramagnetic while [ Ni(CN) 4 ] 2– is diamagnetic - CHEMSOLVE.NET | Electron configuration, Electrons, System](https://i.pinimg.com/736x/f5/9d/33/f59d330381a7793a315e8fcce2d90f54.jpg)
NiCl4] 2– is paramagnetic while [ Ni(CN) 4 ] 2– is diamagnetic - CHEMSOLVE.NET | Electron configuration, Electrons, System
Supporting Information for “Relevant electronic interactions related to the coordination chemistry of tetracyanometallates. An
![Diamagnetic - Paramagnetic - Ni(CO)4, [Ni(CN)4]2- and [NiCl4]2- IIT JEE ... | Chemistry lessons, Chemistry, Lesson Diamagnetic - Paramagnetic - Ni(CO)4, [Ni(CN)4]2- and [NiCl4]2- IIT JEE ... | Chemistry lessons, Chemistry, Lesson](https://i.pinimg.com/originals/a2/20/10/a22010863c43298057a81f0eb09dd692.jpg)
Diamagnetic - Paramagnetic - Ni(CO)4, [Ni(CN)4]2- and [NiCl4]2- IIT JEE ... | Chemistry lessons, Chemistry, Lesson
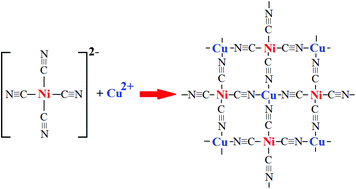
Chemistry and structure by design: ordered CuNi(CN)4 sheets with copper(ii) in a square-planar environment - Dalton Transactions (RSC Publishing)

![Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube](https://i.ytimg.com/vi/5S_my6-2Vkc/maxresdefault.jpg)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)


![Calculate the oxidation number of Ni in `K_(2)[Ni(CN)_(4)]` . - YouTube Calculate the oxidation number of Ni in `K_(2)[Ni(CN)_(4)]` . - YouTube](https://i.ytimg.com/vi/lE5DlvdO6mo/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGHIgRyg8MA8=&rs=AOn4CLB9uPuRTP_m4WJieYIYhi9QclCWfg)
![Solved Tetracyanonickelate(II), INi(CN)4]2 is a square | Chegg.com Solved Tetracyanonickelate(II), INi(CN)4]2 is a square | Chegg.com](https://media.cheggcdn.com/media%2F54e%2F54ef6548-6c25-4904-badf-967cfab1219a%2Fimage)
![Give reason for the statement. $??Ni(CN)_{4}]^{2-} Give reason for the statement. $??Ni(CN)_{4}]^{2-}](https://images.collegedunia.com/public/qa/images/content/2022_07_06/C3A5CUsers_d05d15b31657086194203.png)
![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)
![Deduce the structure of [NiCi 4 ] 2- and [Ni(CN) 4 ] 2- considering t - askIITians Deduce the structure of [NiCi 4 ] 2- and [Ni(CN) 4 ] 2- considering t - askIITians](https://www.askiitians.com/onlinetest/ForumsImages/235-849_8989898.jpg)
![Calculate the difference in magnetic moment of complexes [ Ni CN 4]2 and [ Ni Cl 4]2 . Calculate the difference in magnetic moment of complexes [ Ni CN 4]2 and [ Ni Cl 4]2 .](https://search-static.byjusweb.com/question-images/byjus/infinitestudent-images/ckeditor_assets/pictures/662819/original_B.jpg)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)


![The $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ ion, whic | Quizlet The $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ ion, whic | Quizlet](https://slader-solution-uploads.s3.amazonaws.com/3c5ed73f-36c2-4238-a711-7a222089df1a-1644081443307676.png)
![Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:ffa0132b7d904ae4be8cc8b622fc0250.png)