2. All atoms, except the | Download Scientific Diagram Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig5/AS:667921089052683@1536256205687/Representation-of-the-unit-cell-of-NiH2O6NO32-All-atoms-except-the.jpg)
Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx1.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect
![IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O )6](SO4)2 IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O )6](SO4)2](https://journals.iucr.org/e/issues/2020/03/00/xi2016/xi2016scheme1.gif)
IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O )6](SO4)2
2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar Figure 2 from FIR Spectra of [Ni(H2O)6](ClO4)2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ecaf6b0fee69dc0a5060cdb8d7e5d0c7d395a06e/3-Figure2-1.png)
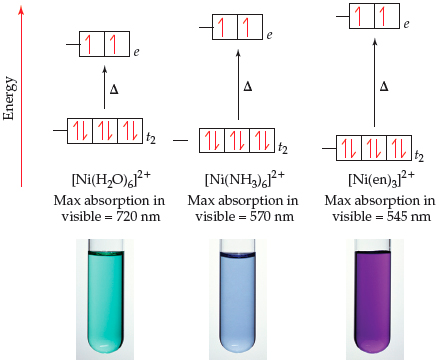

![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://d1hhj0t1vdqi7c.cloudfront.net/v1/SFk3ZV9DMWxyZ28=/sd/)

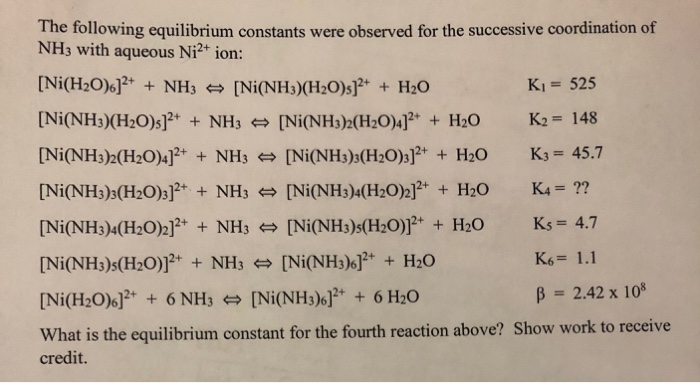
![Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com](https://media.cheggcdn.com/study/284/28408777-e2bb-4645-8742-fab9e76a7424/image)

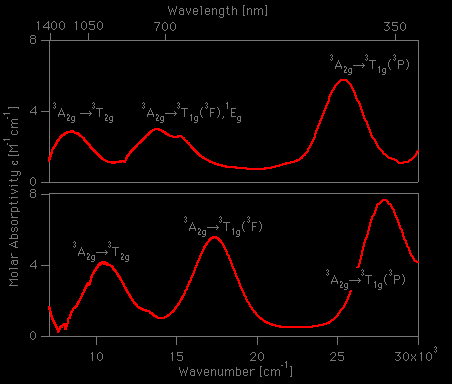
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)

2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022024814007805-gr3.jpg)

![Theoretical Study of [Ni (H2O)n]2+(H2O)m (n ≤ 6, m ≤ 18) | The Journal of Physical Chemistry A Theoretical Study of [Ni (H2O)n]2+(H2O)m (n ≤ 6, m ≤ 18) | The Journal of Physical Chemistry A](https://pubs.acs.org/cms/10.1021/jp108503e/asset/images/medium/jp-2010-08503e_0015.gif)
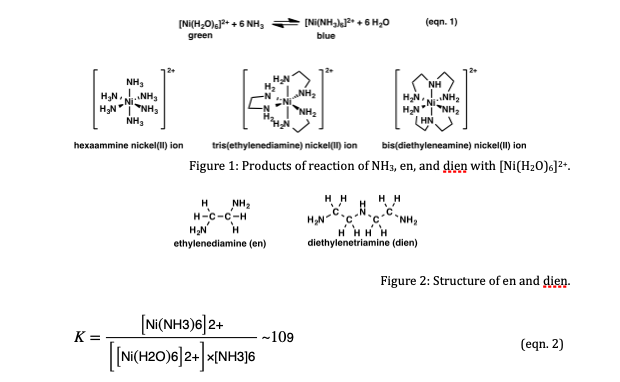
![Solved Part A hexaaquanickel(II) chloride 0 [Ni(H2O)4]C1 O | Chegg.com Solved Part A hexaaquanickel(II) chloride 0 [Ni(H2O)4]C1 O | Chegg.com](https://media.cheggcdn.com/study/c24/c24e28cb-e04a-4e49-8a9a-d56810e6983e/image)
