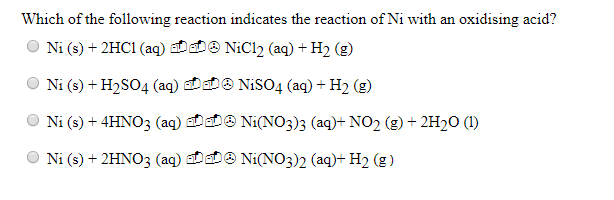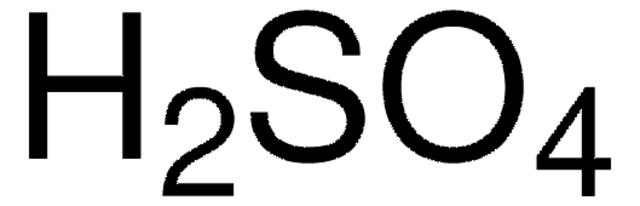A Little Nickel Goes a Long Way: Ni Incorporation into Rh2P for Stable Bifunctional Electrocatalytic Water Splitting in Acidic Media | ACS Materials Au

a LSVs for the HER on the bare Ni foam (a) and NiBTC/Ni foam (b) in... | Download Scientific Diagram
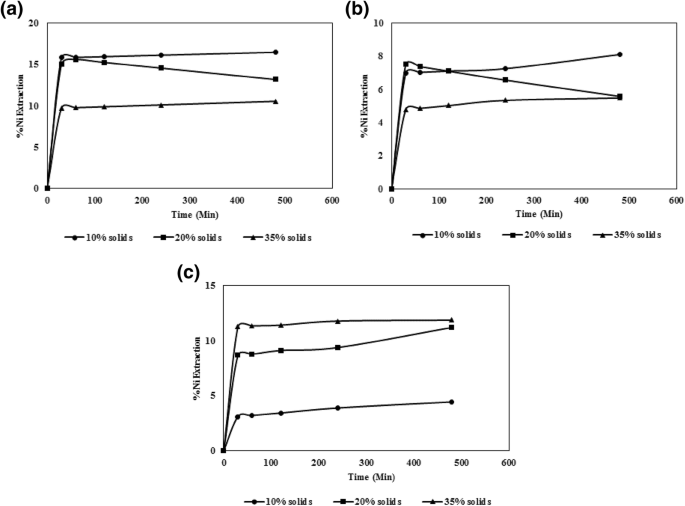
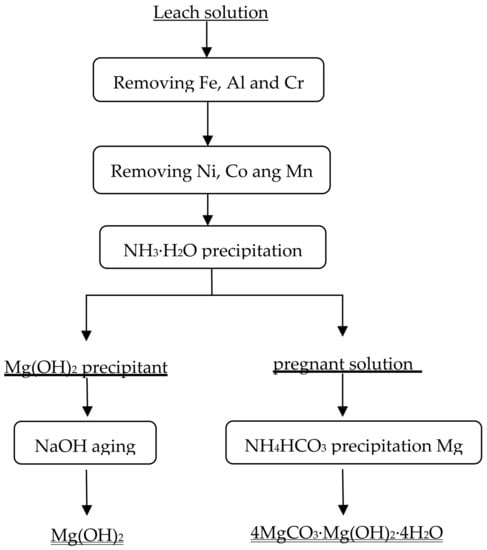
Characteristics of Leaching of Nickel from a Mafic Overburden in Sulfuric Acid and Sodium Chloride Medium at Atmospheric Pressure | SpringerLink

Synergistic Recovery of Valuable Metals from Spent Nickel–Metal Hydride Batteries and Lithium-Ion Batteries | ACS Sustainable Chemistry & Engineering

Ni+H2SO4=H2+Ni2(SO4)3 Balanced Equation||Nickel+Sulphuric acid=Hydrogen+Nickel sulphate Balanced Equ - YouTube

Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure - ScienceDirect

a HER polarization curves in 1 M H2SO4 for chalcogenide gel on Ni foam,... | Download Scientific Diagram

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink

How to balance Ni+H2SO4=Ni2(SO4)3+H2|Chemical equation Ni+H2SO4=Ni2(SO4)3+H2| Ni+H2SO4=Ni2(SO4)3+H2 - YouTube

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink