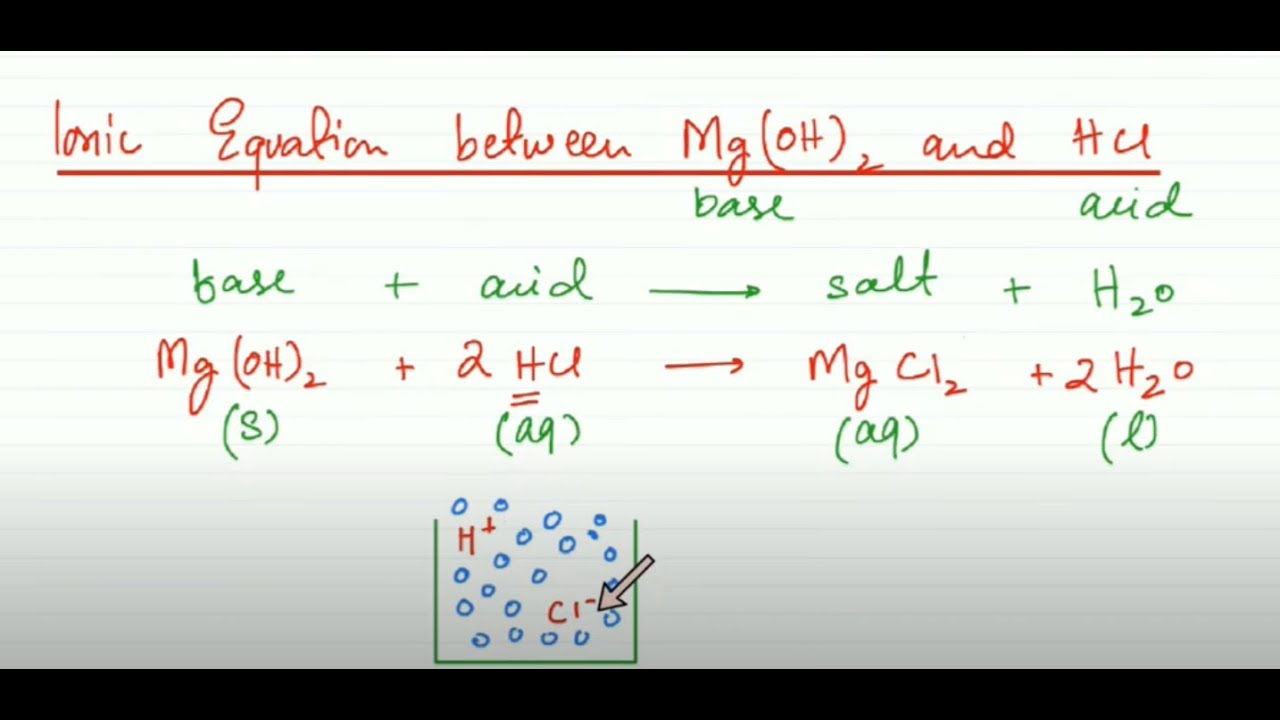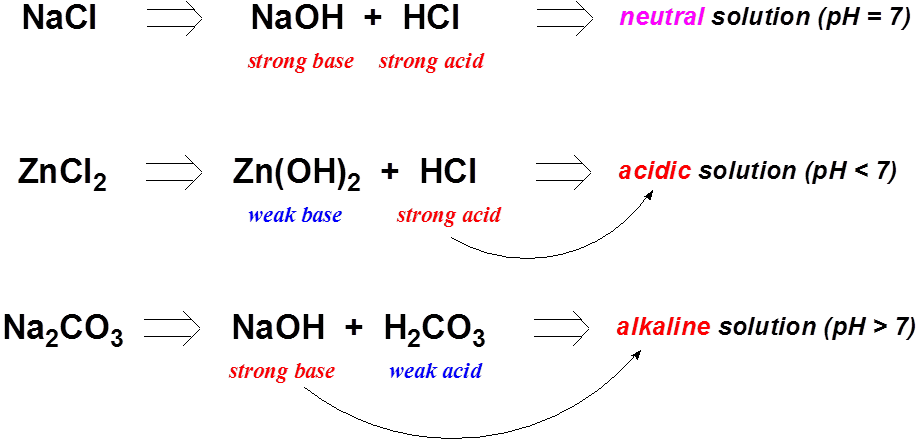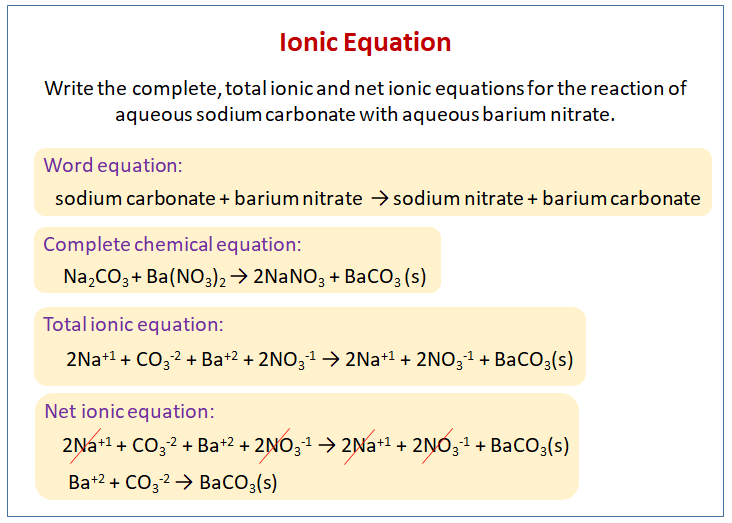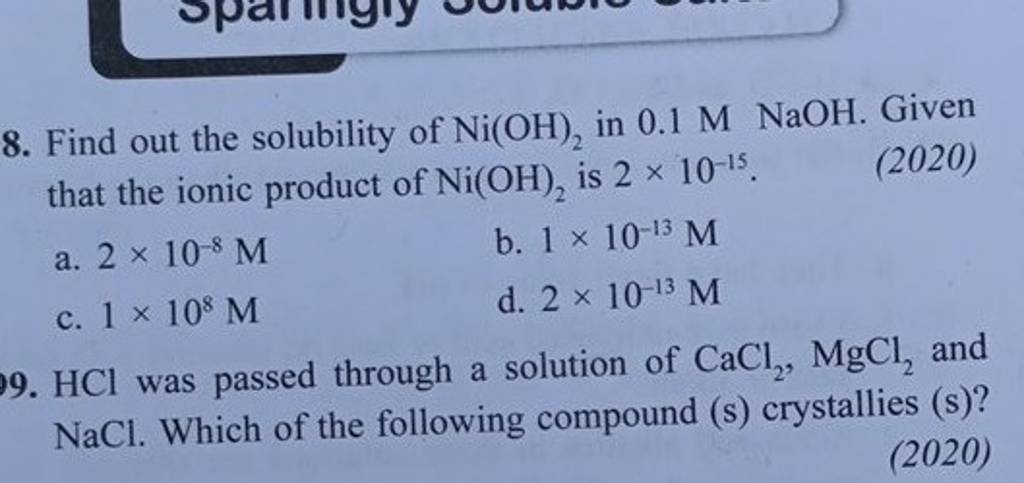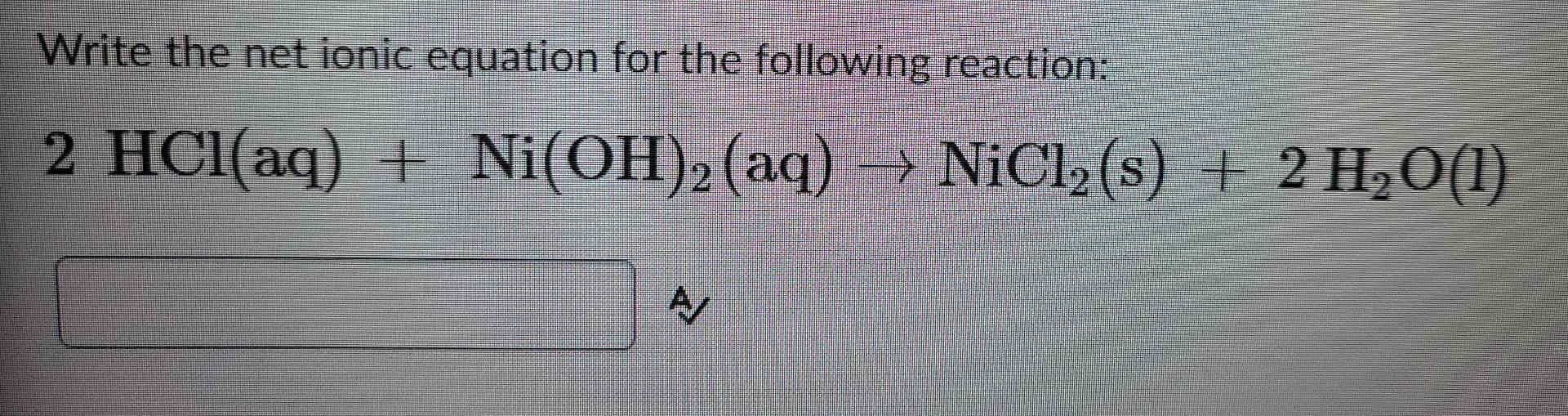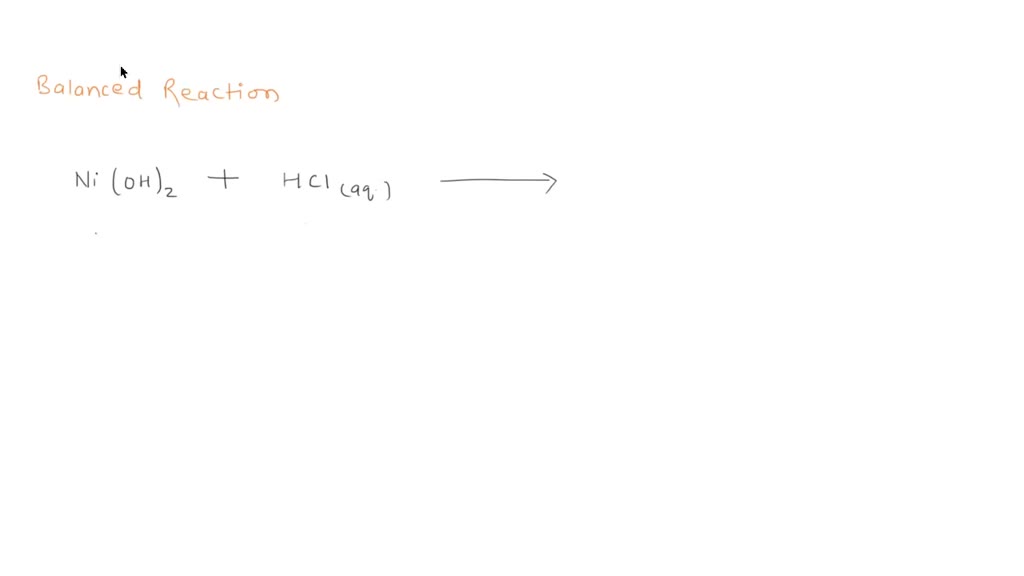
SOLVED: Finely ground nickel (II) hydroxide is placed in a beaker of water. It sinks to the bottom of the beaker and remains unchanged. An aqueous solution of hydrochloric acid is then
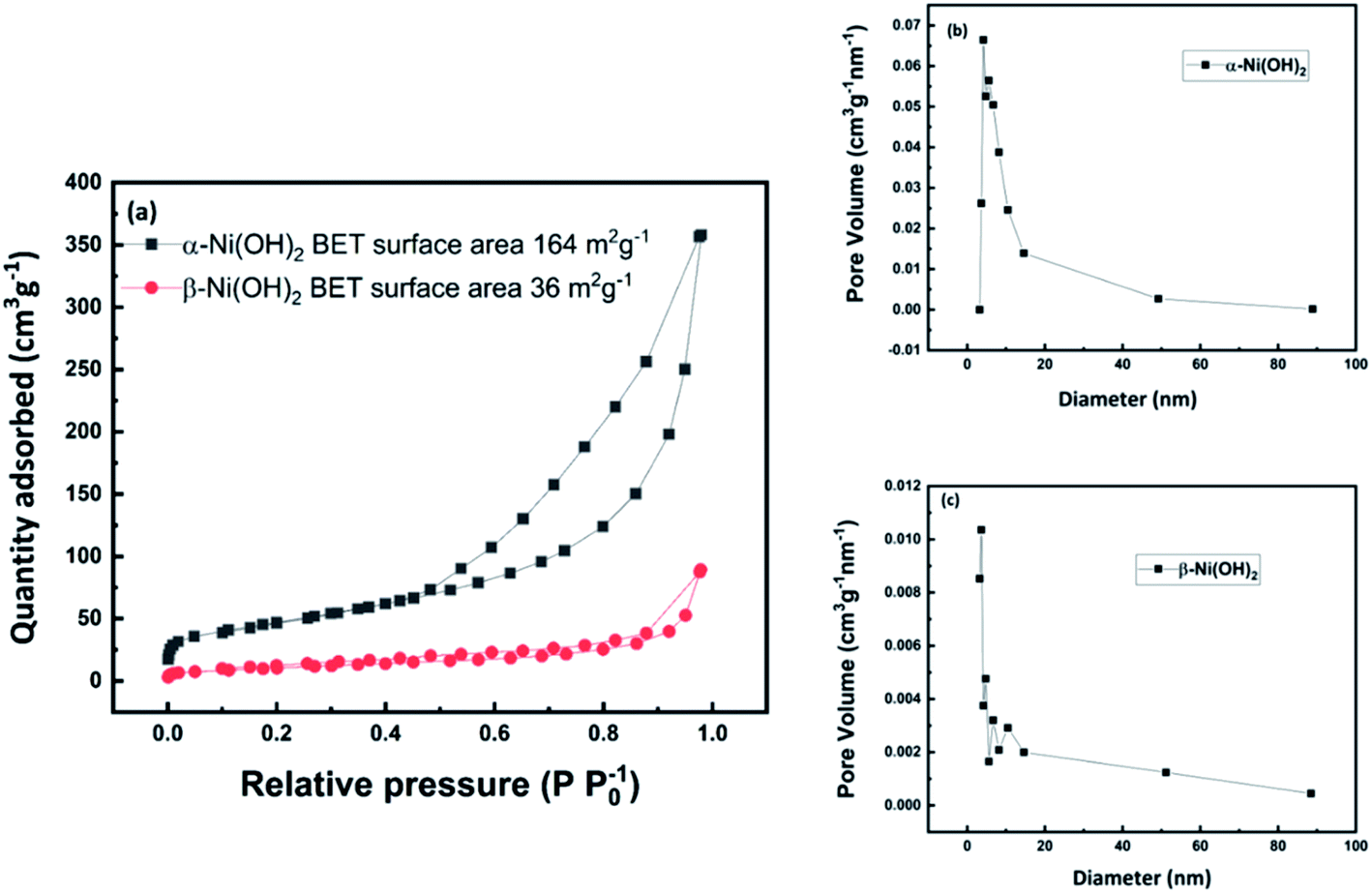
Nanoflower Ni(OH) 2 grown in situ on Ni foam for high-performance supercapacitor electrode materials - Sustainable Energy & Fuels (RSC Publishing) DOI:10.1039/D1SE01036K

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports

Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports

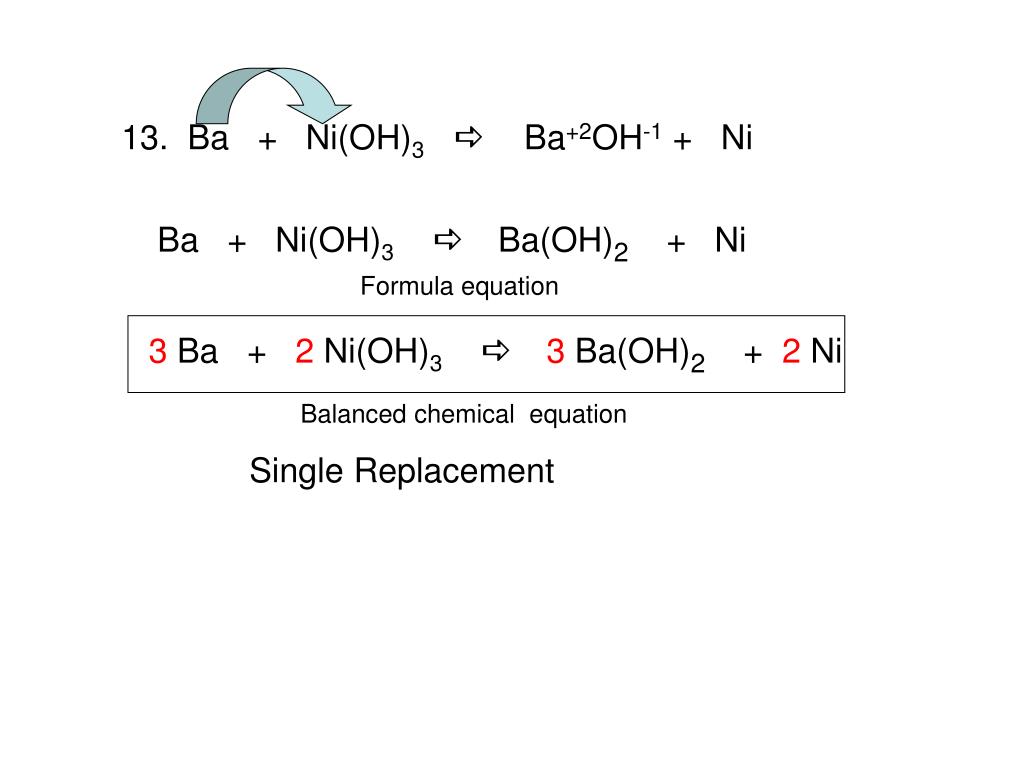
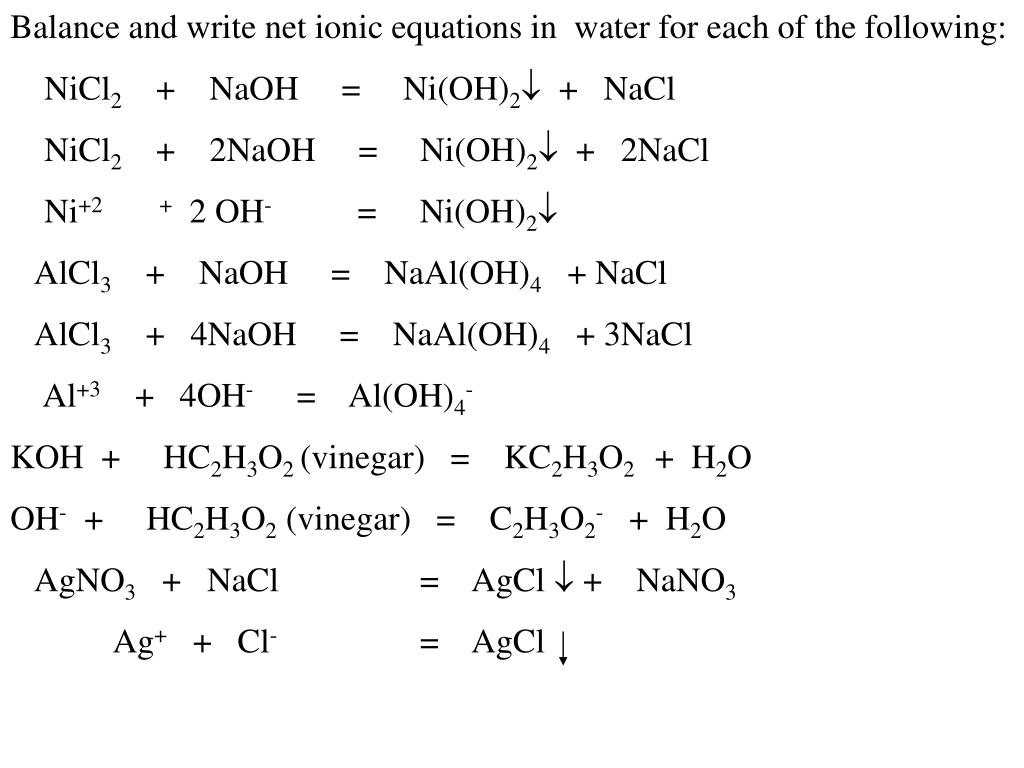
Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download

Electrochemical partial reduction of Ni(OH)2 to Ni(OH)2/Ni via coupled oxidation of an interfacing NiAl intermetallic compound for robust hydrogen evolution - ScienceDirect
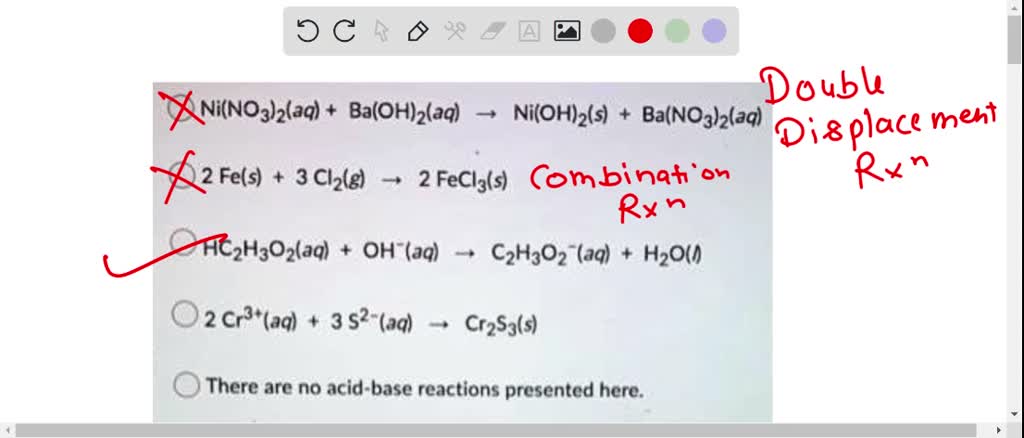
SOLVED: Question 14 (1 point) Which of the following reactions could be classified as an acid-base reaction? Ni(NO3)2(aq) + Ba(OH)2(aq) â†' Ni(OH)2(s) + Ba(NO3)2(aq) 2 Fe(s) + 3 Cl2(g) â†' 2 FeCl3(s)